Morpholinyl dendrimer phthalocyanine: synthesis, photophysical properties and photoinduced intramolecular electron transfer†
Abstract
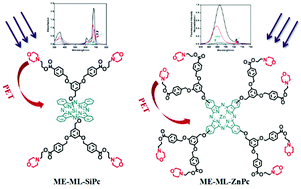
In this work, a novel series of morpholinyl dendrimer phthalocyanines were synthesized. Their structures were characterized by 1H NMR, IR, elemental analysis, ESI-MS as well as MALDI-TOF MS. The photophysical properties of these phthalocyanines were studied by UV/Vis and fluorescence spectroscopic methods. The photophysical properties of these dendrimer phthalocyanines exhibited dependence on the number of morpholinyl groups and the central ion. The photoinduced intramolecular electron transfer from the morpholinyl groups to the phthalocyanine ring was evidenced by the remarkably quenched fluorescence intensity and shortened lifetime of the silicon phthalocyanine. This difference in photoinduced intramolecular electron transfer effect for axially and peripherally substituted morpholinyl dendrimer phthalocyanines was also studied. Besides, introduction of morpholinyl groups on the dendrimer structure could enhance water solubility as well as increase the singlet oxygen quantum yield.


 Please wait while we load your content...
Please wait while we load your content...