Efficient hydroboration of carbonyls by an iron(ii) amide catalyst†
Abstract
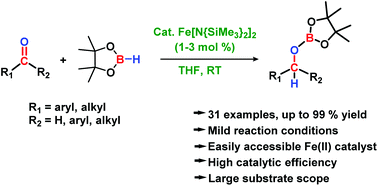
An easily prepared iron(II) amide precatalyst enables the selective hydroboration of carbonyls with HBpin (pinacolborane) in the absence of any additive. The reactions proceed with low catalytic loading (1–3 mol%) under mild reaction conditions and display wide functional group compatibility. Aldehydes are selectively hydroborated in the presence of other reducible functional groups, such as ketones, alkenes, nitriles, esters, amides, acids and halides.


 Please wait while we load your content...
Please wait while we load your content...