Oxide/sulfide-based hybrid arrays as robust electrocatalysts for water splitting†
Abstract
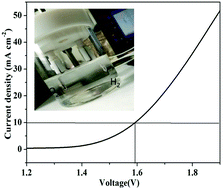
It is imperative to develop bifunctional electrocatalysts with good activity and stability for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). In this paper, a hierarchical NiCo2O4/Ni3S2 hybrid is synthesized by direct growth on nickel foam (NF) using co-precipitation and sulfuration as a general two-step method. When the NiCo2O4/Ni3S2/NF material is used as an electrode, it displays high activity and perfect stability for OER. A low overpotential of only 360 mV is obtained at 40 mA cm−2, comparable to the benchmark of IrO2 electrodes (330 mV overpotential at 40 mA cm−2), benefiting from the particular hybrid structure of NiCo2O4/Ni3S2/NF with large surface area and fast electron transfer. In addition, the NiCo2O4/Ni3S2/NF sample also reveals a superior elevated HER activity compared to NiCo2O4/NF and NF catalysts individually, for which a low overpotential of only 143 mV is obtained at 10 mA cm−2. Beyond that, NiCo2O4/Ni3S2/NF is also used as a bifunctional water splitting catalyst, for which a very low cell voltage of 1.58 V is acquired at 10 mA cm−2 in 1.0 M KOH. The results reveal that oxide/sulfide-based materials can be used as a perfect electrode candidate and afford the advantage of the synergy strategy, which opens a new route toward desired water splitting electrochemical devices of high activity and environmentally friendly electrode materials.


 Please wait while we load your content...
Please wait while we load your content...