Enhanced photocatalytic conversion and selectivity of nitrate reduction to nitrogen over AgCl/TiO2 nanotubes†
Abstract
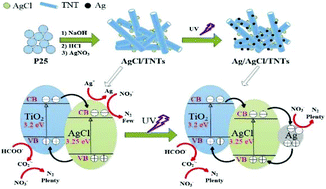
TiO2 nanotubes (TNTs) and AgCl/TiO2 nanotubes (AgCl/TNTs) were synthesized by a hydrothermal method and used in the reduction of nitrate with formic acid as a hole scavenger. As-synthesized photocatalysts were well characterized by field emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), X-ray photoelectron spectra (XPS), N2 adsorption–desorption and UV-vis diffuse reflectance spectroscopy (UV-vis DRS). 5 wt% AgCl/TNTs showed excellent performance in the reduction of nitrate (94.5% conversion of nitrate and 92.9% selectivity to N2, respectively) under UV light (365 nm) irradiation for 30 min. After four reduction cycles of nitrate the 5 wt% AgCl/TNTs also exhibited steady photoactivity. The enhanced photoreductive ability and stability attributed to the nanotube structures provided a higher specific surface area and more active sites. For the combination of AgCl and TNTs, the SPR effect of Ag0 formed by UV light irradiation improved the separation of electron–hole pairs which was proved by the electrochemistry impedance spectra. The effects of AgCl content, hole scavengers, and concentration of formic acid were systematically investigated. Based on the above results a mechanism was proposed. This work provides a novel method for high conversion and selectivity of nitrate reduction to nitrogen by photocatalytic technology.


 Please wait while we load your content...
Please wait while we load your content...