Hydrogenation of titanocene and zirconocene bis(trimethylsilyl)acetylene complexes†
Abstract
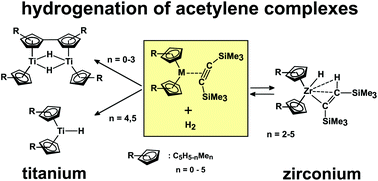
Reactions following the addition of dihydrogen under maximum atmospheric pressure to bis(trimethylsilyl)acetylene (BTMSA) complexes of titanocenes, [(η5-C5H5−nMen)2Ti(η2-BTMSA)] (n = 0, 1, 3, and 4) (1A–1D), and zirconocenes, [(η5-C5H5−nMen)2Zr(η2-BTMSA)] (n = 2–5) (4A–4D), proceeded in diverse ways and, depending on the metal, afforded different products. The former complexes lost, in all cases, their BTMSA ligand via its hydrogenation to bis-1,2-(trimethylsilyl)ethane when reacted at 80 °C for a prolonged reaction time. For n = 0, 1, and 3, the titanocene species formed in situ dimerised via the formation of fulvalene ligands and two bridging hydride ligands, giving known green dimeric titanocenes (2A–2C). For n = 4, a titanocene hydride [(η5-C5HMe4)2TiH] (2D) was formed, similarly to the known [(η5-C5Me5)2TiH] (2E) for n = 5; however, in contrast to this example, 2D in the absence of dihydrogen spontaneously dehydrogenated to the known Ti(III)–Ti(III) dehydro-dimer [{Ti(η5-C5HMe4)(μ-η1:η5-C5Me4)}2] (3B). This complex has now been fully characterised via spectroscopic methods, and was shown through EPR spectroscopy to attain an intramolecular electronic triplet state. The zirconocene-BTMSA complexes 4A–4D reacted uniformly with one hydrogen molecule to give Zr(IV) zirconocene hydride alkenyls, [(η5-C5H5−nMen)2ZrH{C(SiMe3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(SiMe3)}] (n = 2–5) (5A–5D). These were identified through their 1H and 13C NMR spectra, which show features typical of an agostically bonded proton,
CH(SiMe3)}] (n = 2–5) (5A–5D). These were identified through their 1H and 13C NMR spectra, which show features typical of an agostically bonded proton, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(SiMe3). Compounds 5A–5D formed equilibria with the BTMSA complexes 4A–4D depending on hydrogen pressure and temperature.
CH(SiMe3). Compounds 5A–5D formed equilibria with the BTMSA complexes 4A–4D depending on hydrogen pressure and temperature.


 Please wait while we load your content...
Please wait while we load your content...