Synthesis and hydroformylation evaluation of Fréchet-type organometallic dendrons with N,O-salicylaldimine Rh(i) complexes at the focal point†
Abstract
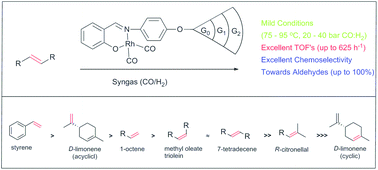
A series of organometallic dendrons containing N,O-salicylaldimine entities at the focal point were synthesised by reacting the N,O-salicylaldimine-functionalised Fréchet dendrons (G0, G1 and G2) with a [Rh(μ-Cl)(η2:η2-COD)]2 dimer to yield the corresponding Rh(COD) [COD = cyclooctadiene] complexes. These Rh(COD) complexes were exposed to an atmosphere of CO to yield a new class of rhodium carbonyl organometallic dendrons with Rh(CO)2 units at the focal point. All the compounds were characterised using standard spectroscopic and analytical techniques, these include nuclear magnetic resonance, infrared spectroscopy, mass spectrometry and single-crystal X-ray diffraction for compounds 1, 4 and 7. All of the complexes were evaluated in the hydroformylation of 1-octene, with excellent conversion and chemoselectivity towards aldehydes. The G0-(CO)2 catalyst precursor (7) was active in the hydroformylation of 1-octene, styrene, 7-tetradecene, methyl oleate, triolein, D-limonene and R-citronellal. The conversion and chemoselectivity towards aldehydes for 7-tetradecene, methyl oleate, triolein and D-limonene were promising. Across a particular dendron series, an increase in chemoselectivity was observed due to the dendritic effect. Mercury drop tests were performed for the G0-analogues and these confirm that the hydroformylation can be attributed to a combination of homogeneous and heterogeneous catalysis.


 Please wait while we load your content...
Please wait while we load your content...