Oxidatively stable ferrocenyl-π-bridge-titanocene D–π-A complexes: an electrochemical and spectroscopic investigation of the mixed-valent states†
Abstract
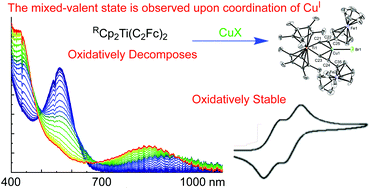
The synthesis, spectroscopic, and electrochemical characterization of oxidatively stable D–π-A compounds of the form (Me2CpC2Fc)2TiCl2 and RCp2Ti(C2Fc)2CuX (where Fc = ferrocenyl) are reported. Oxidative stability enabled by the addition of CuX is evidenced by voltammagrams of the RCp2Ti(C2Fc)2CuX compounds which all display two chemically-reversible 1e− FeIII/II couples, indicative of electronic communication between the Fc- termini. Differential pulse voltammetry (DPV) in CH2Cl2/[n-Bu4N][PF6], demonstrated that the redox potential difference between the two 1e− FeIII/II couples (ΔE1/2) is between 112 mV and 146 mV, being most pronounced with the electron rich Cp*2Ti(C2Fc)2CuBr. The ΔE1/2 values were unaffected by solvent (THF) and displayed only a small dependence on the identity of the counterion, either PF6− or B(C6F5)4−. For each complex with a measurable ΔE1/2 value, spectroelectrochemical experiments were performed in CH2Cl2/[n-Bu4N][PF6] and gave clear evidence of both the one-electron oxidized mixed-valent (MV) state and the two-electron oxidized state, each with distinct spectroscopic signatures. The MV states of these complexes showed absorbance between 820 and 940 nm which were replaced with a higher energy feature following a second oxidation. A very similar absorption band was also observed in the one-electron oxidized state of an analogue with only a single Fc substituent, namely TMSCp2Ti(C2Fc)(C2Ph)CuBr, suggesting this feature is not an FeII/FeIII intravalence charge-transfer (IVCT) band. Despite DFT calculations suggesting a pathway exists for electronic coupling, NIR spectroscopy on the MV states gave no evidence of an FeII/FeIII IVCT. Possible contributions to ΔE1/2 from inductive effects and a superexchange mechanism are discussed.


 Please wait while we load your content...
Please wait while we load your content...
