Reactivity studies of an imine-functionalised phosphaalkene; unusual electrostatic and supramolecular stabilisation of a σ2λ3-phosphorus motif via hydrogen bonding†
Abstract
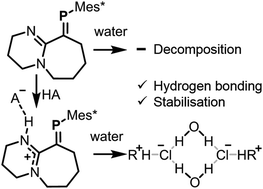
A P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C heavy-alkene analogue that is unreactive towards the addition of strong acids on its double-bond is presented; instead, a strategically located imine nitrogen on the periphery forms protonated adducts displaying hydrogen bonding interactions. These materials are significantly more stable than the parent species, demonstrating an unprecedented approach towards the stabilisation of a multiple-bonded heavy main group fragment, in this case, a phosphaalkene. An HCl adduct self-assembles with H2O into a dimeric network displaying a discrete quadrilateral hydrogen-bonded arrangement.
C heavy-alkene analogue that is unreactive towards the addition of strong acids on its double-bond is presented; instead, a strategically located imine nitrogen on the periphery forms protonated adducts displaying hydrogen bonding interactions. These materials are significantly more stable than the parent species, demonstrating an unprecedented approach towards the stabilisation of a multiple-bonded heavy main group fragment, in this case, a phosphaalkene. An HCl adduct self-assembles with H2O into a dimeric network displaying a discrete quadrilateral hydrogen-bonded arrangement.
- This article is part of the themed collection: New Talent: Europe


 Please wait while we load your content...
Please wait while we load your content...