On the alcoholysis of alkyl-aluminum(iii) alkoxy-NHC derivatives: reactivity of the Al-carbene Lewis pair versus Al-alkyl†
Abstract
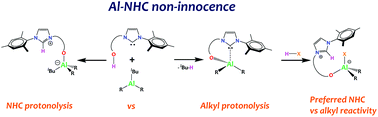
The reaction of a bifunctional hydroxy N-heterocyclic carbene (NHC-OH) ligand with alkyl-aluminum(III) derivatives appears to be dependent on the precursor used. The expected alkoxy-NHC metallated product is indeed obtained with Al(iBu)3. In contrast, the sterically hindered [Al(iBu)(OAr)2] (OAr = 2,6-di-tert-butyl-4-methylphenoxy) displays reactivity at the carbene and affords an imidazolium-aluminate zwitterion. The non-innocence of the Al–NHC motif is further highlighted by the heterolytic cleavage of the phenol O–H bond across the Al–CNHC bond from Al(O-NHC)X2 derivatives (X = iBu, OAr).
- This article is part of the themed collection: New Talent: Europe


 Please wait while we load your content...
Please wait while we load your content...