Mixed azido/phenoxido bridged trinuclear Cu(ii) complexes of Mannich bases: Synthesis, structures, magnetic properties and catalytic oxidase activities†
Abstract
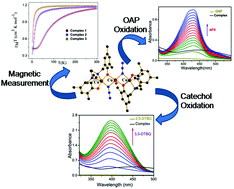
Three similar Mannich base ligands viz. N,N-bis(3,5-dimethyl-2-hydroxybenzyl)-N′,N′-dimethyl-1,3-diaminopropane (H2L1), N,N-bis(3,5-dimethyl-2-hydroxybenzyl)-N′,N′-dimethyl-1,2-diaminoethane (H2L2) and N,N-bis(3,5-dimethyl-2-hydroxybenzyl)-N′,N′-diethyl-1,2-diaminoethane (H2L3) upon reaction with Cu(CH3COO)2·H2O produced dinuclear complexes [Cu2L21–3]. The reaction of each of these isolated dimeric species with Cu(ClO4)2·6H2O and NaN3 resulted in three new trinuclear complexes, [(CuL1)2(μ1,1-N3)2Cu(H2O)]·CH3OH (1), [(CuL2)2(μ1,1-N3)2Cu(H2O)]·CH3OH (2) and [(CuL3)2(μ1,1-N3)2Cu(H2O)]·2CH3OH (3), respectively. The complexes (1–3) have been characterized by elemental analysis and single crystal X-ray diffraction. In all three complexes, the central Cu(II) ion is coordinated by two terminal [CuL] units through a phenoxido and an azido bridge. These are the first trinuclear Cu(II) complexes of this type of Mannich base ligands. Magnetic susceptibility measurements showed intramolecular antiferromagnetic interactions with J = −64.42, −9.60 and −4.54 cm−1 for 1, 2 and 3, respectively. All three complexes exhibited catecholase-like and phenoxazinone synthase-like activities towards the aerobic oxidation of 3,5-di-tert-butylcatechol and o-aminophenol, respectively. The turnover numbers (kcat) for the aerobic oxidation of 3,5-di-tert-butylcatechol are 568.8, 542.1 and 500.4 h−1 and those of o-aminophenol are 125.83, 118.9 and 114.7 h−1 for complexes 1–3, respectively. The X-band EPR spectroscopy and estimation of the produced hydrogen peroxide indicated that the aerobic oxidation of 3,5-di-tert-butylcatechol proceeded through the formation of a semiquinonate radical. The mechanism of phenoxazinone synthase-like activities is also proposed for trinuclear Cu(II) catalysts with the help of mass spectral analysis.


 Please wait while we load your content...
Please wait while we load your content...