Facile incorporation of technetium into magnetite, magnesioferrite, and hematite by formation of ferrous nitrate in situ: precursors to iron oxide nuclear waste forms
Abstract
The fission product, 99Tc, presents significant challenges to the long-term disposal of nuclear waste due to its long half-life, high fission yield, and to the environmental mobility of pertechnetate (TcO4−), the stable Tc species in aerobic environments. Migration of 99Tc from disposal sites can potentially be prevented by incorporating it into durable waste forms based on environmentally stable minerals. Since Tc(IV) and Fe(III) have the same ionic radius, Tc(IV) can replace Fe(III) in iron oxides. Environmentally durable iron oxides include goethite (α-FeOOH), hematite (α-Fe2O3), and magnesioferrite (MgFe2O4). The incorporation of Tc into two of these, hematite and magnesioferrite, as well as magnetite (Fe3O4) by means of simple, aqueous chemistry is presented starting from TcO4− in 5 M nitric acid. A combination of X-ray diffraction and X-ray absorption fine structure spectroscopy reveals that Tc(IV) replaces Fe(III) within the iron oxide structures. Following incorporation, Tc doped samples were suspended in deionized water under aerobic conditions, and the release rates of Tc were determined. The results of this work show that Tc leaches more quickly from Fe3O4 than from α-Fe2O3 or MgFe2O4. Modeling the leach rates and comparison with the leach rate of Tc from TiO2 indicate that release of Tc is controlled by solid state diffusion.
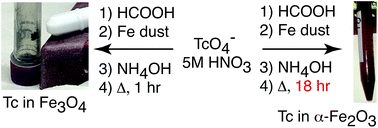


 Please wait while we load your content...
Please wait while we load your content...
