Detection of sulfide ion and gaseous H2S using a series of pyridine-2,6-dicarboxamide based scaffolds†
Abstract
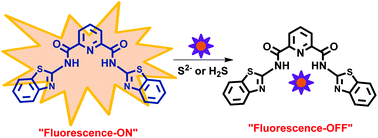
Pyridine-2,6-dicarboxamide based scaffolds with different appendages act as chemosensors for the selective detection of S2− ion, as well as gaseous H2S, in primarily aqueous media. Out of nine synthesized chemosensors, one with benzothiazole ring appendages was found to be highly selective for S2− ion and gaseous H2S. A series of spectroscopic studies confirmed that sulfide ion abstracts amidic N–H groups thus leading to the in situ generation of HS− ion, which remain bound to the pincer cavity of the resultant anionic chemosensor, and it was found that acetic acid could be used to reverse this process. It was essential for a chemosensor to feature a pincer cavity to recognize sulfide ion, whereas the sensing mechanism involved the deprotonation of the amidic N–H groups. We also illustrate the detection of sulfide ions and gaseous H2S in live cells and paper-strip sensing applications.


 Please wait while we load your content...
Please wait while we load your content...