From monomeric complexes to double-stranded helicates constructed around trans-TiO4N2 motifs with intramolecular inter-ligand hydrogen-bonding interactions†
Abstract
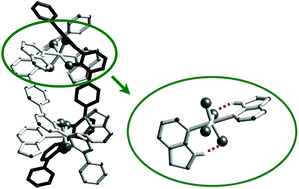
A coordination chemistry involving trans-TiO4N2 motifs is described, where the oxygen ligands are 2,2′-biphenolato derivatives (L1 or L2) and the nitrogen ligands are pyridine (Pyr), 2,3-dihydro-7-azaindole (DHA) or 2-(methylamino)pyridine (MePyr). Monomeric complexes and double-stranded helicates incorporating this set of ligands are characterized. The monomeric species are obtained from the precursor [Ti(L1)2(HOiPr)2] whereas the helical dinuclear architectures are synthesized following a multicomponent self-assembly approach starting from L2H4, Ti(OiPr)4 and two equivalents of the nitrogen ligand. It is proposed that the trans isomerism in TiO4N2 observed in these structures results from the destabilisation of the cis isomer by the trans influence of the Ti–N bonds. The crystal structures and infra-red analysis demonstrate hydrogen bonding interactions occurring between the NH group of DHA or MePyr and the oxygens belonging to the titanium coordination sphere. The strength of these interactions is estimated using the PACHA (Partial Atomic Charges Analysis) software.


 Please wait while we load your content...
Please wait while we load your content...