Spontaneous formation of a chiral (Mo2O2S2)2+-based cluster driven by dimeric {Te2O6}-based templates†
Abstract
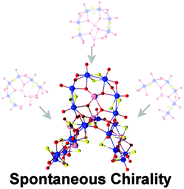
Utilization of [Mo2S2O2(H2O)6]2+ and a tellurite anion led to the formation of three new clusters, 1–3, with unique structural features. The tellurite anion not only templated the formation of [(Mo2O2S2)4(TeO3)(OH)9]3−1 and [(Mo2O2S2)12(TeO3)4(TeO4)2 (OH)18]10−3, but also the in situ generation of two different types of dimeric {Te2O6} based moieties induced the spontaneous assembly of the chiral [(Mo2O2S2)10(TeO3)(Te2O6)2(OH)18]8− anionic cluster, 2.


 Please wait while we load your content...
Please wait while we load your content...