Synthesis and non-conventional structure of square-planar Pd(ii) and Pt(ii) complexes with an N,C,N-chelated stibinidene ligand†
Abstract
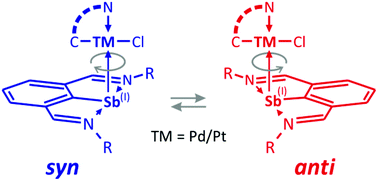
The N,C,N-chelated stibinidene, ArSb (Ar = C6H3-2,6-(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NtBu)2), reacts with Pt(II) compounds [PtCl2L2] resulting in the formation of 1 : 1 complexes, cis-[PtCl2L(ArSb)] (L = Me2S (1), dmso (2)). In contrast, attempts to synthesize similar Pd(II) complexes failed, resulting only in the formation of elemental palladium. To increase the stability of the ArSb complexes, in particular those containing Pd(II), the simple auxiliary ligands were replaced with C,N-chelating ones, which led to a set of four compounds of the type [RMCl(ArSb)], where R = C6H4-2-(CH2NMe2) or Fe(η5-C5H4)(η5-C5H3-2-(CH2NMe2)) and M = Pd (3, 5) or Pt (4, 6). Compounds 1–6 were characterized by 1H and 13C{1H} NMR spectroscopy and single-crystal X-ray diffraction analysis, and in the case of ferrocene derivatives 5 and 6, also by cyclic voltammetry. Compounds 2–6 were shown to form rotamers in solution due to the side-on coordination of the ArSb ligand and a hindered rotation around the Sb–Pd(Pt) bond. This process was investigated by 1H-VT-NMR spectroscopy and by DFT computations.
NtBu)2), reacts with Pt(II) compounds [PtCl2L2] resulting in the formation of 1 : 1 complexes, cis-[PtCl2L(ArSb)] (L = Me2S (1), dmso (2)). In contrast, attempts to synthesize similar Pd(II) complexes failed, resulting only in the formation of elemental palladium. To increase the stability of the ArSb complexes, in particular those containing Pd(II), the simple auxiliary ligands were replaced with C,N-chelating ones, which led to a set of four compounds of the type [RMCl(ArSb)], where R = C6H4-2-(CH2NMe2) or Fe(η5-C5H4)(η5-C5H3-2-(CH2NMe2)) and M = Pd (3, 5) or Pt (4, 6). Compounds 1–6 were characterized by 1H and 13C{1H} NMR spectroscopy and single-crystal X-ray diffraction analysis, and in the case of ferrocene derivatives 5 and 6, also by cyclic voltammetry. Compounds 2–6 were shown to form rotamers in solution due to the side-on coordination of the ArSb ligand and a hindered rotation around the Sb–Pd(Pt) bond. This process was investigated by 1H-VT-NMR spectroscopy and by DFT computations.


 Please wait while we load your content...
Please wait while we load your content...