Multi-molecular emission of a cationic Pt(ii) complex through hydrogen bonding interactions†
Abstract
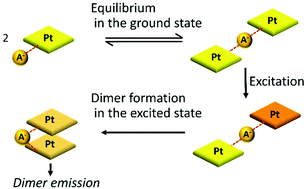
The cationic Pt complexes with amide groups have been found to show dimer emission through hydrogen bonding interactions with counter anions even at low concentration. In order to investigate further details of dimer emission, we prepared three Pt complexes, Pt·B(C6F5)4, Pt·Cl, and Pt·PF6, whose counter anions possess different strengths of a hydrogen bonding acceptor. Hydrogen bonding interactions in the ground state and excited-state dynamics of the Pt complexes were evaluated by NMR analysis, temperature dependence, and kinetics of dimer emission. These studies revealed that the hydrogen bonding interaction in the ground state is essential for dimer emission, but too strong hydrogen bonding prevents dimer emission due to the inhibition of a stacked dimer formation in the excited state. Owing to this trade-off, the Pt complex with a moderate hydrogen bonding acceptor, PF6−, most effectively shows dimer emission. In general, a strong supramolecular interaction efficiently provides a desired assembled structure showing multi-molecular emission. We revealed a unique phenomenon that a moderate interaction is beneficial to effective multi-molecular emission.


 Please wait while we load your content...
Please wait while we load your content...