Adsorptive removal of organic dyes from aqueous solution by a Zr-based metal–organic framework: effects of Ce(iii) doping†
Abstract
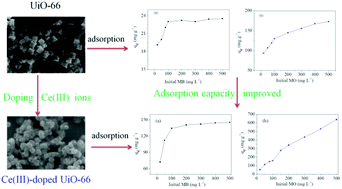
Herein, we report the synthesis and characterization of Ce(III)-doped UiO-66 nanocrystals, revealing their potential to efficiently remove organic dyes such as methylene blue (MB), methyl orange (MO), Congo red (CR), and acid chrome blue K (AC) from aqueous solutions. Specifically, the room-temperature adsorption capacities of Ce(III)-doped UiO-66 equaled 145.3 (MB), 639.6 (MO), and 826.7 (CR) mg g−1, exceeding those reported for pristine UiO-66 by 490, 270, and 70%, respectively. The above behavior was rationalized based on zeta potential and adsorption isotherm investigations, which revealed that Ce(III) doping increases the number of adsorption sites and promotes π–π interactions between the adsorbent and the adsorbate, thus improving the adsorption capacity for cationic and anionic dyes and overriding the effect of electrostatic interactions. The obtained results shed light on the mechanism of organic dye adsorption on metal–organic frameworks, additionally revealing that the synergetic interplay of electrostatic, π–π, and hydrophobic interactions results in the operation of two distinct adsorption regimes depending on adsorbate concentration.


 Please wait while we load your content...
Please wait while we load your content...