Carbodiphosphorane-based nickel pincer complexes and their (de)protonated analogues: dimerisation, ligand tautomers and proton affinities†
Abstract
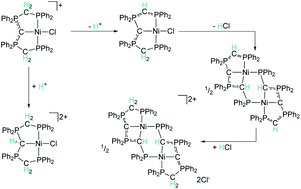
The reactivity patterns of carbodiphosphoranes (CDPs) as ligands are much less explored than those of isoelectronic analogues. In the current manuscript, we investigate the reactivity of the carbodiphosphorane-based PCP nickel(II) pincer complex [({dppm}2C)NiCl]Cl (1) towards acids and bases, calculate proton affinities, analyse the bonding situation and tautomeric forms with the aim to evaluate whether CDPs can potentially act as cooperative ligands in catalysis (dppm = 1,1-bis(diphenylphosphino)methane). Our investigations show that different tautomeric forms are stable for the coordinated and the uncoordinated ligand. The protonated CDP-based complex 2 represents a rare example of a cationic donor group binding to a cationic metal centre. The continuous arm-deprotonation of 1 leads to the formation of remarkably stable dimers with Ni–C–P–C-metallacycles. In comparison to corresponding boron and amine-based ligands, the coordinated CDP-group exhibits the lowest proton affinity according to DFT calculations, indicating that coordinated CDP ligands can potentially serve as proton relay in cooperative catalysis.
- This article is part of the themed collection: New Talent: Europe


 Please wait while we load your content...
Please wait while we load your content...