Alkyl chain elongation and acyl group effects in a series of Eu/Tb complexes with hexadentate π-electronic skeletons and their enhanced luminescence in solutions†
Abstract
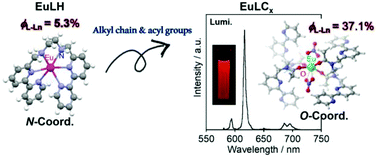
Five Eu complexes with long alkyl chain groups, abbreviated as EuLCx (“x” indicates the number of methylene groups: x = 8, 12, 14, 18, and 22), were synthesized to evaluate their structural and luminescence properties in chloroform. The mother helicate Eu complex, EuL, which has two bipyridine moieties bridged by an ethylenediamine, has been previously reported. A reduced form in which the azomethine groups of L also coordinated to the Eu ion, EuLH, was newly prepared. EuLH also adopts a helicate molecular structure based on single crystal X-ray structural analysis. The amine hydrogens of the bridging ethylenediamine of LH are active sites for substitution and were exchanged with five different alkyl chains to form EuLCx. Luminescence band positions and shapes of EuLCx in chloroform were completely identical, with a quantum yield of 37.1 ± 1.2 and a lifetime of around 1.25 ms. This indicates that the environments surrounding the Eu ion in the various complexes are all similar. Luminescence quantum yields of TbLH and TbLC18 are also strengthened, 48.7% in acetonitrile and 55% in chloroform, respectively. Potential energy surfaces were also described by using density functional theory, suggesting the possibility of a 1 : 2 complex of Eu and the ligand as a main luminescent species in solutions. This 1 : 2 complexation forms Eu–oxygen coordination using acyl groups. It indicates that the acyl group modification results in a different structure from the mother complexes.


 Please wait while we load your content...
Please wait while we load your content...