Destruction of chemical warfare agent simulants by air and moisture stable metal NHC complexes†
Abstract
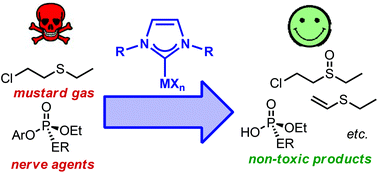
The cooperative effect of both NHC and metal centre has been found to destroy chemical warfare agent (CWA) simulants. Choice of both the metal and NHC is key to these transformations as simple, monodentate N-heterocyclic carbenes in combination with silver or vanadium can promote stoichiometric destruction, whilst bidentate, aryloxide-tethered NHC complexes of silver and alkali metals promote breakdown under mild heating. Iron–NHC complexes generated in situ are competent catalysts for the destruction of each of the three targetted CWA simulants.


 Please wait while we load your content...
Please wait while we load your content...