Luminescence properties of mechanochemically synthesized lanthanide containing MIL-78 MOFs
Abstract
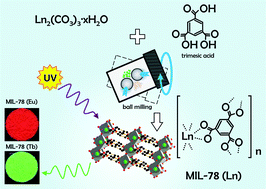
Three metal–organic framework (MOF) compounds, Ln0.5Gd0.5{C6H3(COO)3}; Ln = Eu, Tb, and Dy with a MIL-78 structure, have been synthesized by a solvent-free mechanochemical method from stoichiometric mixtures of benzene 1,3,5-tricarboxylic acid, C6H3(COOH)3, also known as trimesic acid, and the respective lanthanide carbonates, Ln2(CO3)3·xH2O, Ln = Eu, Gd, Tb and Dy. MIL-78 (Ln0.5Gd0.5) shows the characteristic red, green, and yellow luminescence of Eu3+, Tb3+, and Dy3+, respectively. Efficient intramolecular energy transfer from the ligand triplet state to the excited states of Ln3+ ions can be observed. The lifetimes and quantum yields of these compounds are studied and discussed in detail. Among the three compounds, the Tb3+ containing compound shows the longest lifetime and highest quantum yield due to a smaller contribution from non-radiative decay pathways and better matching of the lowest triplet energy level of the benzenetricarboxylate ligand and the resonance level of Tb3+.


 Please wait while we load your content...
Please wait while we load your content...