Ferrimagnetism in manganese-rich gallium and aluminium spinels due to mixed valence Mn2+–Mn3+ states
Abstract
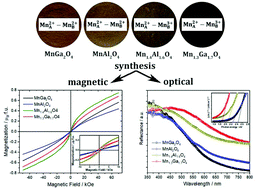
Stoichiometric (MnGa2O4 and MnAl2O4) and Mn-rich (Mn1.3Ga1.7O4 and Mn1.4Al1.6O4) spinels with a small inversion degree (0.14–0.21) were obtained via a co-precipitation route followed by calcination of the as-synthesized coprecipitates at 700–1000 °C under different gas atmospheres (air, N2 or argon). In situ synchrotron XRD at elevated temperatures reveals the conditions for synthesizing phase-pure materials. The stoichiometry of the samples is confirmed by inductively coupled plasma optical emission spectrometry as well as by structure refinement of neutron diffraction data of phase-pure specimens. XANES characterization reveals the average oxidation state of manganese to be +2.2 and 2.3 in Mn1.3Ga1.7O4 and Mn1.4Al1.6O4 spinels, respectively. The mixed Mn2+–Mn3+ valence states are responsible for the ferrimagnetic properties of Mn1.3Ga1.7O4 and Mn1.4Al1.6O4 samples below 48 and 55 K, respectively, as well as for a smaller optical bandgap when compared to stoichiometric spinels.


 Please wait while we load your content...
Please wait while we load your content...