Structural evolution and stability of Sc2(WO4)3 after discharge in a sodium-based electrochemical cell†
Abstract
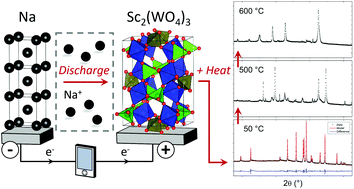
Sc2(WO4)3, prepared by solid state synthesis and constructed as an electrode, is discharged to different states in half-cell batteries, versus a Na negative electrode. The structural evolution of the Na-containing electrodes is studied with synchrotron powder X-ray diffraction (PXRD) revealing an increase in microstrain and a gradual amorphization taking place with increasing Na content in the electrode. This indicates that a conversion reaction takes place in the electrochemical cell. X-ray absorption spectroscopy (XAS) at the tungsten L3 absorption edge shows a reduction in the tungsten oxidation state. Variable temperature (VT) PXRD shows that the Sc2(WO4)3 electrode remains relatively stable at higher temperatures, while the Na-containing samples undergo a number of phase transitions and/or turn amorphous above ∼400 °C. Although, Sc2(WO4)3 is a negative thermal expansion (NTE) material only a subtle change of the thermal expansion is found below 400 °C for the Na-containing electrodes. This work shows the complexity in employing an electrochemical cell to produce Na-containing Sc2(WO4)3 and the subsequent phase transitions.


 Please wait while we load your content...
Please wait while we load your content...