Synthetic routes to a coordinatively unsaturated ruthenium complex supported by a tripodal, protic bis(N-heterocyclic carbene) phosphine ligand†
Abstract
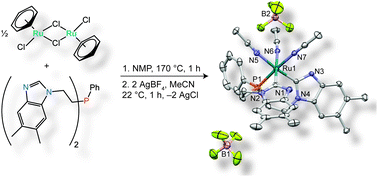
A facile, one pot synthesis of a coordinatively unsaturated ruthenium complex supported by a tripodal, protic bis(N-heterocyclic carbene) phosphine ligand is presented. A number of coordination complexes were discovered en route during this synthesis, revealing some of the unique aspects of complexes ligated by this type of tridentate, protic bis(NHC) ligand. Through a combination of 1D and 2D NMR spectroscopic analysis and single crystal X-ray diffraction, we reveal the intermediacy of phosphine-ligated bisimidazole complexes and show that abstraction of inner-sphere halide ions facilitates conversion to the desired tridentate bis(NHC) coordination mode. Ultimately the use of N-methyl-2-pyrrolidone is shown to enable the use of the extreme temperatures needed to facilitate the direct, thermally activated tautomerization reaction that gives rise to the bis(NHC) motif.


 Please wait while we load your content...
Please wait while we load your content...