Supramolecular frameworks based on 5,10,15,20-tetra(4-carboxyphenyl)porphyrins†
Abstract
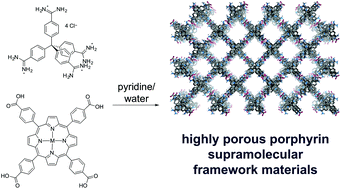
We have investigated the hydrogen bond-driven assembly of nickel and freebase tetra(carboxyphenyl) and tetra(carboxylatophenyl) porphyrins. When the tetracarboxylates were crystallized with bis(amidinium) species, their crystal structures contained a range of hydrogen bond geometries, and we did not obtain well-ordered networks. Use of a tetrahedral tetra(amidinium) building block yielded a 3D framework material with a PtS topology, which contains only a “paired” hydrogen bonding arrangement. This framework is highly porous, with ∼3/4 of the unit cell volume occupied by disordered solvent molecules, although it loses crystallinity upon removal from solvent. Favourable interactions between porphyrin carboxylic acid hydrogen bond donors and bipyridine nitrogen atoms were then used to prepare a stable 2D porphyrin grid-like network.


 Please wait while we load your content...
Please wait while we load your content...