Spectroscopy as a tool to detect multinuclear Cu(ii)–triethanolamine complexes in aqueous solution†
Abstract
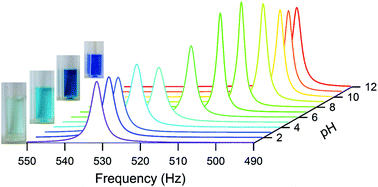
Complexes of Cu(II) with triethanolamine (TEA) are widely used in aqueous precursor solutions of Cu-based catalysts and metal oxides such as YBa2Cu3O7−δ superconductors. An outstanding question is whether such complexes are multinuclear in solution. Here, we use various spectroscopic techniques to unmistakably prove the existence of such multimers. Firstly, we introduce an original approach based on NMR spectroscopy and the Evans method that establishes the existence of multimers in aqueous solution at pH 4 and higher, and allows precise monitoring of the formation of these complexes with increasing pH. Secondly, we use extended X-ray absorption fine structure (EXAFS) spectroscopy to show that a Cu–Cu interaction exists at pH 9.5, which is not observed in acidic (pH 2) solutions. Finally, NMRD measurements reveal additional structural information regarding the multinuclear complexes. Knowledge concerning the nature of Cu(II)–TEA complexes in solution is of great relevance in view of the design of speciation models to predict the stability of copper triethanolamine-based precursor solutions.


 Please wait while we load your content...
Please wait while we load your content...