In situ synthesis of novel Cu2CO3(OH)2 decorated 2D TiO2 nanosheets with efficient photocatalytic H2 evolution activity
Abstract
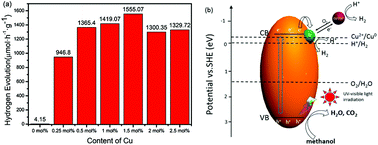
Semiconductor-based photocatalytic hydrogen evolution from water with earth-abundant and low cost co-catalysts has attracted much attention. Herein, novel Cu2(OH)2CO3 decorated 2D TiO2 nanosheets for photocatalytic water splitting were synthesized by a facile in situ synthetic method. The chemical and photophysical properties of Cu2(OH)2CO3/TiO2 nanosheets were investigated by X-ray diffractometry (XRD), transmission electron microscopy (TEM), UV-vis diffusion reflectance spectroscopy (DRS), X-ray photoelectron spectroscopy (XPS) and cyclic voltammetry (CV) analysis. At an optimal Cu2(OH)2CO3 loading content of 1.5 mol%, the hybrid photocatalyst delivers a high photocatalytic H2 production rate of 1555.07 μmol g−1 h−1. Such enhanced photocatalytic activity is attributed to tight interfaces formed between Cu2(OH)2CO3 nanoparticles and TiO2 nanosheets, which play a vital role in the separation of photo-excited carriers, and the formation of active Cu1+ and Cu0 species can also benefit the charge separation process by reducing the over-potential of water reduction. Based on the above results, a possible mechanism is proposed and further verified using photoluminescence (PL) spectra. This work may provide more insight into the synthesis of novel Cu2(OH)2CO3/TiO2 nanosheets with high photocatalytic H2 evolution activity for solar-to-chemical conversion and utilization.


 Please wait while we load your content...
Please wait while we load your content...