Synthesis and comparison of nickel, palladium, and platinum bis(N-heterocyclic carbene) pincer complexes for electrocatalytic CO2 reduction†
Abstract
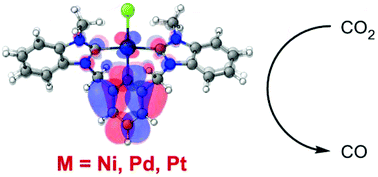
A valence isoelectronic and isostructural series of charged bis(N-heterocyclic carbene) pincer complexes [M(bC^N^bC)X]OTf and [M(bC^N^bC)CH3CN](OTf)2 (where M = Ni, Pd, and Pt, bC^N^bC = 1,1′-(pyridine-2,6-diylbis(methylene))bis(3-butylbenzo[d]imidazol-2-ylidene)) were synthesized, characterized, modelled by density functional theory calculations, and compared for their electrochemical properties and reactivity with CO2. Although the electrochemical response of each complex is altered by the presence of CO2, controlled potential electrolysis experiments demonstrated the superior ability of [Pd] to reduce CO2 to CO in faradaic efficiencies up to 58% in the presence of trifluoroacetic acid, compared to [Pt] and [Ni] which showed only marginal production of CO, giving the trend [Pd] ≫ [Pt] > [Ni] for this series.


 Please wait while we load your content...
Please wait while we load your content...