Synthesis, characterization and Pd(ii)-coordination chemistry of the ligand tris(quinolin-8-yl)phosphite. Application in the catalytic aerobic oxidation of amines†‡
Abstract
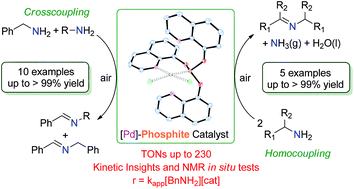
The synthesis and characterization of the ligand tris(quinolin-8-yl)phosphite, (P(Oquin)3), are described and its coordination chemistry toward the metal precursor [Pd(COD)Cl2] (COD = 1,5-cyclooctadiene, C8H12) is reported. A new Pd(II)–P(Oquin)3 metal complex was isolated and fully characterized ([Pd{P(Oquin)3}Cl2]), and its X-ray diffraction analysis shows that the phosphite ligand coordinates as a bidentate P–N chelate. This complex is an efficient catalyst for the solvent-free mild oxidative coupling of primary amines to imines using air as an oxidant, obtaining moderate to good yields (up to 99%) and turnover numbers (TONs up to 230). This catalyst can be recovered from the reaction mixture and reused in a subsequent run without a significant loss of activity. Kinetic measurements of the oxidation of benzylamine suggest that the rate law is r = kapp[BnNH2][cat] ([BnNH2] = molar concentration of benzylamine, [cat] = molar concentration of [Pd{P(Oquin)3}Cl2], kapp = k[O2]c = 0.756 L mol−1 h−1 = apparent rate constant). In situ NMR tests were performed to gain some insight into the reactivity of the Pd(II)–P(Oquin)3 complex toward benzylamine.


 Please wait while we load your content...
Please wait while we load your content...