Design, synthesis, and evaluation of nickel dipyridylmethane complexes for Coordination-Induced Spin State Switching (CISSS)†
Abstract
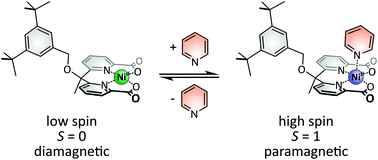
In order to develop a new system capable of performing Ligand-Driven Coordination-Induced Spin State Switching (LD-CISSS), a new CISSS complex based on a nickel(II) dipyridylmethane framework was rationally designed, synthesized, and investigated both experimentally and theoretically. In DFT calculations, a dipyridylmethane dicarboxylic acid ligand was determined to be most suitable for this purpose. The corresponding nickel(II) complex was synthesized by an optimized procedure, and the CISSS effect was evidenced by crystallography as well as Evans NMR susceptibility measurements. In the solid state, the square planar nickel complex is diamagnetic and the octahedral bispyridine adduct is paramagnetic. Also, a dimer containing three ethanol ligands was obtained. Dimer formation occurs in solution as well. Upon addition of pyridine, a continuous transition from predominantly diamagnetic to paramagnetic behavior takes place. Equilibrium constants for the binding of pyridine as well as corresponding thermodynamic parameters indicate a high affinity for this ligand and show that the title system is a suitable candidate for LD-CISSS.


 Please wait while we load your content...
Please wait while we load your content...