Copper(i) complexes based on ligand systems with two different binding sites: synthesis, structures and reaction with O2†
Abstract
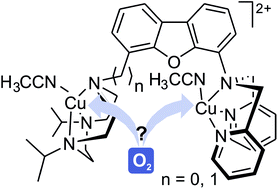
The synthesis of the ligand systems L1 and L2 with two different N3-binding sites linked through a dibenzofuran spacer and their coordination properties towards a variety of CuI precursors are reported. The reaction of L1 with copper halides leads to the formation of a bimetallic species [(L1)(CuICl)2] (1), and metallodimers [((L1)(CuIX)2)2(μ-(CuI2)(μ-X)2)] (2: X = Br, 3: X = I) in which two dicopper complexes are bridged by a (μ-(CuI2)(μ-X)2)-moiety whereas L2 reacts with copper chloride to afford {[CuI2(L2)Cl2]}n (8). Furthermore, starting from L1 in combination with copper(I) salts of weakly coordinating anions the dicopper complexes [(L1)(CuI(NCCH3))2](BF4)2 (4), [(L1)(CuI(NCCH3))(Cu(Y))](Y) (5: Y = OTf, 6: Y = ClO4) and [(L1)(CuI2(dppe))](PF6)2 (7) were isolated, and employing L2, the complexes [(L2)(CuI(NCCH3))2](Z)2 (9: Z = PF6, 10: Z = OTf) and [(L2)(CuI2(dppe))](PF6)2 (11) were obtained. Complexes 4–6 as well as 9 and 10 react rapidly with O2 to form metastable O2 adducts in acetone at −90 °C, where O2 is bound between the two copper centers within one dicopper molecule, as evidenced by UV/Vis spectroscopy, kinetic investigations, Raman spectroscopy and studies with ligands containing the isolated donor sites. The reactivity of the O2 adducts towards selected substrates was also investigated, showing their ability to act as electrophiles as well as nucleophiles.


 Please wait while we load your content...
Please wait while we load your content...