Tunable ligand emission of napthylsalophen triple-decker dinuclear lanthanide(iii) sandwich complexes†
Abstract
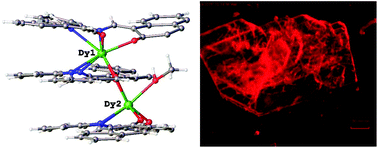
The fluorescent ligand 1,1′-((1E,1′E)-(1,2-phenylenebis(azanylylidene))bis(methanylylidene)) bis(naphthalen-2-ol) (H2L) was used to prepare lanthanide(III) metal complexes. These were found to self-assemble as triple decker sandwich complexes of the type (Ln2L3), where Ln = Pr(III), Nd(III), Sm(III), Eu(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Yb(III), or Lu(III). The structures of the complexes Nd2L3, Gd2L3, Tb2L3, Dy2L3, Ho2L3, Yb2L3, and Lu2L3 are structurally characterized by single crystal X-ray diffraction. In the Nd2L3 complex, both metals are 8 coordinate. Yb2L3, Tb2L3, Dy2L3, and Lu2L3 are isostructural. In these, as in the Gd2L3 and Ho2L3 complexes, one metal is 8 coordinate, one 7 coordinate. The ligand was found to have tunable emission in the solid state across the lanthanide series with a maximum at 556 nm for the Sm2L3 complex to 617 nm for Er2L3. Of these, most demonstrate only ligand-centered fluorescence at room temperature. The ligand was found to have much greater fluorescence in the complex Lu2L3. Here, we describe these distinctive triple decker complexes and their absorption and emission properties as both solids and solutions.


 Please wait while we load your content...
Please wait while we load your content...