Selective and reversible adsorption of cationic dyes by mixed ligand Zn(ii) coordination polymers synthesized by reactant ratio modulation†
Abstract
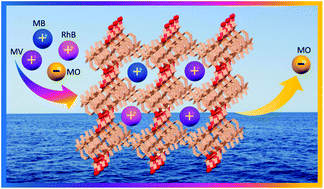
Dye capture and separation through coordination polymers (CPs) has been a promising research field in recent times due to the toxic and nondegradable nature of organic dyes released into the environment from various industries as well as the reusability of CPs for the said purpose. Here, we report the synthesis and characterization of two mixed ligand CPs {[Zn2(HBTC)2(L)(H2O)2](C2H5OH)3}n (CP1) and {[Zn5(BTC)2(L)3(OH)4(H2O)2](H2O)4(CH3OH)11}n (CP2) (where H3BTC = 1,3,5-benzene tricarboxylic acid and L = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene) by the stoichiometric variation of the precursors. The crystal structure analysis revealed that CP1 is a 2D network composed of a [Zn2(HBTC)2(H2O)2]n motif linked via terminal nitrogen atoms of L and CP2 is a 3D framework in which symmetrically disposed two-dimensional {[Zn5(BTC)2(L)3(OH)4(H2O)2]}n sheets composed of pentanuclear [Zn5(RCO2)6(μ3-OH)2(μ2-OH)2(H2O)2] SBUs are pillared by L ligands. Adsorption and separation of cationic dyes by CP1 and the solid-state fluorescence properties of both CPs have been investigated. Cationic dyes (RhB, MB, and MV) can be effectively adsorbed by CP1 from their aqueous solution (61%, 90%, and 97%, respectively) while the anionic dye methyl orange (MO) remains uncaptured. Dye desorption studies and CP1 as a column chromatographic filler for the separation of cationic dyes in water have also been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...