DFT methods applied to answer the question: how accurate is the ligand acidity constant method for estimating the pKa of transition metal hydride complexes MHXL4 when X is varied?†
Abstract
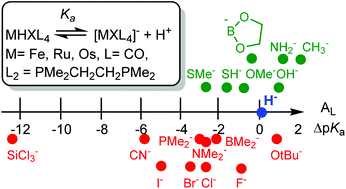
Density functional theory (DFT) is used to calculate the relative free energies of deprotonation of the isomers of iron-group hydride complexes MHXL4 where M = Fe, Ru, Os, L4 = (CO)4 or (PMe2CH2CH2PMe2)2 for a wide range of anionic ligands X. The free energies of the most stable isomers are used to calculate relative pKa values where Ka refers to the acid dissociation constant for the equilibrium MHXL4 → [MXL4]− + H+. These are used to test the proposal that the pKa for a given metal complex in THF can be simply calculated by adding the contributions to the total pKa value from each ligand L; these are called ligand acidity constants (LAC) AL used in the LAC equation [R. H. Morris, J. Am. Chem. Soc., 2014, 136, 1948–1959]. The AL are calculated using AL = 0.2 as a reference for the hydride ligand. The AL of certain less polarizable X ligands are found to be fairly constant (±1 to ±2 units) and consistent with the proposed LAC method for a range of the complexes considered: 2 for Me−, 1 for OH−, 1 for NH2−, 0 for B(OCH2CH2O−)−, −1 for OMe−, −2 for SH− and −2 for SMe−. Other ligands have more variable AL values (±3 to ±5 units) because of high polarizability or other reasons: 1 for OtBu−, −1 for F−, −2 for BMe2−, −2 for NMe2−, −3 for Cl−, −3 for PMe2−, −3 for Br−, −5 for I−, −6 for CN− and −12 for SiCl3−. Iodide stabilizes the anion [MIL4]− more than does fluoride in [MFL4]− making iodide the more acidifying ligand despite its lower electronegativity. DFT is also used to validate the charge correction in the LAC equation.


 Please wait while we load your content...
Please wait while we load your content...