Displacement of carbonates in Ca2UO2(CO3)3 by amidoxime-based ligands from free-energy simulations†
Abstract
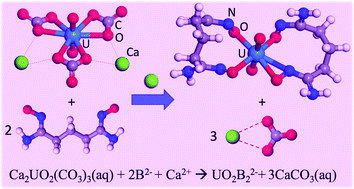
Amidoxime-based ligands are effective in uranium extraction by displacing carbonates in Ca2UO2(CO3)3, the dominant uranyl species in seawater. However, a detailed understanding of the displacement process has been lacking. Here we use classical molecular dynamics combined with umbrella sampling to map the complete displacement process and the free-energy profiles by the simple acetamidoximate (AO−) and the more complex glutardiamidoximate (B2− and HB−) ligands. Interestingly, we find that the two Ca2+ ions in Ca2UO2(CO3)3 can greatly facilitate the displacement of the first two carbonate groups. Displacing the third carbonate is however significantly more uphill than the first two. With the help of an additional Ca2+ ion, the third carbonate displacement can be made less uphill. Comparing AO− and B2−/HB− ligands, we find that the displacement by the latter is thermodynamically more favorable due to the chelate effect. Our free-energy simulations based on classical molecular dynamics simulations reveal key atomistic details and quantify the thermodynamic driving force during the carbonate displacement of Ca2UO2(CO3)3 by amidoxime-based ligands. These findings will be useful in understanding seawater uranium extraction by amidoxime-grafted polymeric sorbents.


 Please wait while we load your content...
Please wait while we load your content...