Stabilization of Ni2+ dimers in hexacyano Mo6 cluster-based Prussian blue derivatives: experimental and theoretical investigations of magnetic properties†
Abstract
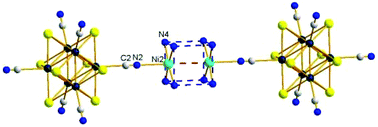
Herein, two new octahedral molybdenum cyanide cluster compounds, namely [{Ni(NH3)6}4{Ni2(NH3)8}1][Mo6Br6Q2(CN)6]3·12H2O, Q = S (1) and Se (2), have been synthesized as single crystals by slow diffusion of a solution of nickel chloride into aqueous ammonia solutions of a K2Cs2[Mo6Br6Q2(CN)6] molybdenum cyanide cluster-based compound. Both 1 and 2 were structurally characterized by single-crystal X-ray diffraction. They are isostructural and crystallize in the cubic system (Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 229); Z = 2, a = 18.147(1) Å, and V = 5976(1) Å3 and a = 18.188(2) Å and V = 6016(2) Å3 for 1 and 2, respectively). 1 and 2 are based on the association of [Mo6Bri6Qi2(CN)a6]4− (Q = S, Se) cluster anions with Ni2+ dimer-based cubic [Ni2(NH3)8]4+ and octahedral [Ni(NH3)6]2+ cations. The structure is based on 2-fold interpenetrated [{Ni(NH3)6}4{Ni2(NH3)8}1][Mo6Br6Q2(CN)6]3 frameworks related to each other by [½, ½, ½] translation. The unit cell is based on a body-centered cubic framework of cubic [Ni2(NH3)8]4+. The [Mo6Bri6Qi2(CN)a6]4− (Q = S, Se) cluster units are located in the middle of the edges and at the center of the faces of the cell. The [{Ni(NH3)6}]2+ cations are located at the center of the cubes of the a/2 edge. The dimers [Ni2(NH3)8]4+ are stabilized by hydrogen bonds between the cyanide ligands of the cluster unit and the hydrogen atoms of the ammonia molecules. Both compounds exhibit a weak antiferromagnetic coupling within the [Ni2(NH3)8]4+ dimer entities at low temperatures together with a paramagnetic behavior originating from the cations of the octahedral [{Ni(NH3)6}]2+ complexes.
m (no. 229); Z = 2, a = 18.147(1) Å, and V = 5976(1) Å3 and a = 18.188(2) Å and V = 6016(2) Å3 for 1 and 2, respectively). 1 and 2 are based on the association of [Mo6Bri6Qi2(CN)a6]4− (Q = S, Se) cluster anions with Ni2+ dimer-based cubic [Ni2(NH3)8]4+ and octahedral [Ni(NH3)6]2+ cations. The structure is based on 2-fold interpenetrated [{Ni(NH3)6}4{Ni2(NH3)8}1][Mo6Br6Q2(CN)6]3 frameworks related to each other by [½, ½, ½] translation. The unit cell is based on a body-centered cubic framework of cubic [Ni2(NH3)8]4+. The [Mo6Bri6Qi2(CN)a6]4− (Q = S, Se) cluster units are located in the middle of the edges and at the center of the faces of the cell. The [{Ni(NH3)6}]2+ cations are located at the center of the cubes of the a/2 edge. The dimers [Ni2(NH3)8]4+ are stabilized by hydrogen bonds between the cyanide ligands of the cluster unit and the hydrogen atoms of the ammonia molecules. Both compounds exhibit a weak antiferromagnetic coupling within the [Ni2(NH3)8]4+ dimer entities at low temperatures together with a paramagnetic behavior originating from the cations of the octahedral [{Ni(NH3)6}]2+ complexes.


 Please wait while we load your content...
Please wait while we load your content...