Cu–Mn composite oxides: highly efficient and reusable acid–base catalysts for the carbonylation reaction of glycerol with urea
Abstract
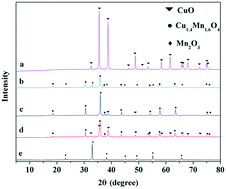
A series of Cu–Mn composite oxides were prepared by co-precipitation. Interestingly, catalysts with varied Cu/Mn molar ratios showed different catalytic performances for glycerol carbonylation. The physicochemical properties of the catalysts are characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy, temperature-programmed desorption (TPD) of CO2 and NH3 and temperature-programmed reduction (H2-TPR) technology. The results showed that the Cu1.4Mn1.6O4 crystal phase is the active component of the catalysts for the carbonylation of glycerol, this phase can effectively provide Mn4+ and lattice oxygen (O2−), and the existence of the Mn4+–O2− Lewis acid–base pair can promote the formation of glycerol carbonate. Various reaction parameters, such as reaction temperature, time, the molar ratio of glycerol to urea and the amount of catalysts, are studied. Under optimizing reaction conditions, the conversion of glycerol is 91.0% with 99.1% glycerol carbonate selectivity.


 Please wait while we load your content...
Please wait while we load your content...