Site-selective C–H bond carbonylation with CO2 and cobalt-catalysis†
Abstract
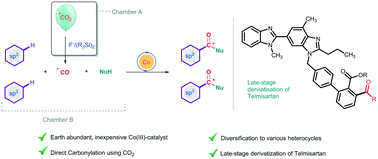
Utilization of anthropogenic greenhouse gas CO2 for catalytic C–C bond formation via conversion to essentially valuable C1 synthons like CO is very challenging. The requirement of an efficient catalyst that has the ability to convert CO2 into CO and activate inert C–H bonds is the bottleneck. We herein demonstrate a tandem approach accomplished in a two-chamber system for efficient fluoride-mediated generation of CO from CO2 using disilane as a deoxygenating reagent and utilization of the in situ-produced CO gas for C–H bond carbonylation using earth-abundant cobalt catalysts. The ease of handling CO2 gas at atmospheric pressure allows us to prepare 13C labelled compounds which are otherwise difficult to achieve. The procedure developed makes it possible to utilize CO2 as a CO source, which can be widely applied as a C1 synthon that can be incorporated between C–H and N–H bonds of aromatic, hetero-aromatic and aliphatic carboxamides for the synthesis of various cyclic imides including spirocycles in a site-selective fashion. The late-stage derivatization of a well-known angiotensin receptor blocker (ARB), Telmisartan, and a well-known drug for very low-density lipoproteins (VLDLs), Gemfibrozil, is demonstrated. Further, to showcase the generality of the reaction, various pharmacologically important and privileged scaffolds like xanthone, coumarin and isatin have been synthesized with CO2 under atmospheric pressure.


 Please wait while we load your content...
Please wait while we load your content...