The effect of potassium on Cu/Al2O3 catalysts for the hydrogenation of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan in a fixed-bed reactor
Abstract
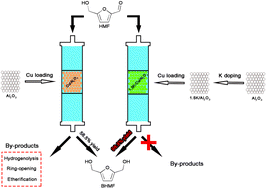
The highly efficient selective hydrogenation of 5-hydroxymethylfurfural (HMF) to 2,5-bis(hydroxymethyl)furan (BHMF) was achieved in a fixed-bed reactor by using inexpensive potassium-doped Cu/Al2O3 catalysts, which were prepared via a successive incipient wetness impregnation method. The characterization results revealed that the introduction of potassium could adjust the size of the copper particles and modify the acid–base property of the catalyst, leading to a significant change in its performance for the selective hydrogenation of HMF to BHMF. The highest yield of BHMF (98.9%) was obtained over the 1.5K–Cu/Al2O3 catalyst under the reaction conditions of 120 °C, 2.0 MPa of H2, and a weight hourly space velocity (WHSV) of 1.0 h−1. The excellent performance could be explained by the fact that the addition of an appropriate amount of potassium facilitated the dispersion of copper and reduced the acidity of the catalyst, which improved the catalytic activity and suppressed unwanted side reactions, respectively.


 Please wait while we load your content...
Please wait while we load your content...