Spectroscopic analysis of proton exchange during the photocatalytic decomposition of aqueous acetic acid: an isotopic study on the product distribution and reaction rate†
Abstract
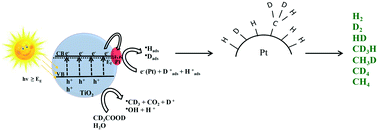
The photocatalytic decomposition of aqueous acetic acid into molecular hydrogen, carbon dioxide, and hydrocarbons employing platinized titania (Pt/TiO2) as a photocatalyst has been studied. In order to investigate the detailed reaction mechanism, isotopic labelling experiments were performed by using solvents (H2O and D2O) and acetic acids (CH3COOH, CD3COOD, and CH3COOD) with different isotopic compositions. The main reaction products resulting from the photocatalytic decomposition of aqueous acetic acid were determined quantitatively in the gas phase via quadrupole mass spectroscopy (QMS). Carbon dioxide, molecular hydrogen, molecular deuterium, and several isotopologues of methane were observed as the main reaction products. The evolution rates of all the main reaction products and their corresponding distribution were found to be strongly influenced by different isotopologues of both acetic acid and water. For instance, the reaction system Pt/TiO2–CD3COOD–D2O yields approximately 2 times higher evolution rates for all main gaseous reaction products in comparison with the reaction system Pt/TiO2–CH3COOH–H2O. Moreover, the data analysis from the isotopic labelling studies illustrates a high proton exchange reaction at both the carboxyl and the methyl group of acetic acid, which is further confirmed by 1H nuclear magnetic resonance (NMR) spectroscopy. For the reaction systems Pt/TiO2–CD3COOD–H2O and Pt/TiO2–CH3COOH–D2O, the NMR spectra demonstrated the transformation of CD3COOD into CD2HCOOD(H) and CH3COOH into CH2DCOOH(D), respectively. This means that during the photocatalytic decomposition of aqueous acetic acid, the protons participating in the formation of molecular hydrogen and methane originate from both acetic acid and water. However, the main source for the molecular hydrogen and methane evolution was found to be the solvent while acetic acid acts as a sacrificial reagent in the overall reaction.


 Please wait while we load your content...
Please wait while we load your content...