Effect of acidity and ruthenium species on catalytic performance of ruthenium catalysts for acetylene hydrochlorination†
Abstract
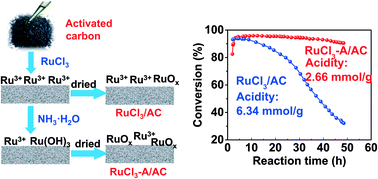
Carbon-supported ruthenium catalysts are promising mercury-free catalysts for acetylene hydrochlorination, due to their high activity and relatively low price. However, ruthenium catalysts often suffer from serious deactivation. Herein, a stable RuCl3-A/AC catalyst was prepared by applying a simple ammonia treatment during the impregnation process. The fresh and used ruthenium catalysts were comprehensively characterized using N2 sorption, NH3-temperature-programmed desorption (NH3-TPD), H2 temperature-programmed reduction (H2-TPR), thermogravimetric analysis (TGA), and X-ray photoelectron spectroscopy (XPS). The results show that the RuCl3 species is identified as the active species, and the surface acidity of the RuCl3/AC catalyst is generated mainly from supported RuCl3 species, which can easily cause coke deposition. The enhancement of the stability of the RuCl3-A/AC catalyst is attributed to the formation of RuOx species and the decrease of the surface acidity.


 Please wait while we load your content...
Please wait while we load your content...