CO2 formation mechanism in Fischer–Tropsch synthesis over iron-based catalysts: a combined experimental and theoretical study†
Abstract
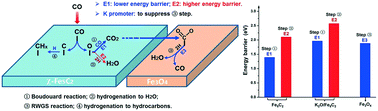
Fischer–Tropsch synthesis (FTS) is one of the most attractive routes to convert syngas (CO + H2) into liquid fuels and high value-added chemicals. However, FTS over Fe-based catalysts generates and emits large amounts of CO2, which reduces the carbon atom economy and causes huge greenhouse gas emission. In this work, CO2 formation mechanisms in FTS over Fe-based catalysts were systematically investigated by combining experiments and DFT calculations, aiming to provide atomic-scale insights into the CO2 formation process. Our results indicate that the Boudouard mechanism, in which the surface O* species formed by CO* dissociation reacts with another CO* to form CO2, plays a predominant role in CO2 formation on the active χ-Fe5C2 phase, while the hydrogenation of surface O* species to form H2O is hindered. The existence of the Fe3O4 phase is favorable for the reverse water-gas shift (RWGS) reaction, leading to the decrease of CO2 selectivity and increase of the amount of generated H2O. The modification by the potassium promoter does not alter the predominant reaction pathway for CO2 formation over Fe-based FTS catalysts and the Boudouard mechanism still plays the dominant role. The potassium promoter can increase CO2 selectivity and decrease the amount of H2O mainly through the following two ways: (1) potassium largely increases the proportion of the χ-Fe5C2 phase and thus increases the amount of active sites for the Boudouard reaction; (2) potassium leads to the disappearance of the Fe3O4 phase and thus suppresses the RWGS reaction. The electronic structures were systematically analyzed to shed light on the nature of the potassium effect. On the one hand, the potassium promoter makes the d-band center of the χ-Fe5C2(510) surface atoms shift toward the Fermi level, facilitating the back-donation of electrons from the χ-Fe5C2(510) surface to the adsorbed CO* antibonding orbital; on the other hand, the direct interaction between K2O and adsorbed CO* weakens the C–O bond by decreasing its electron density, which also contributes to the promoted CO dissociation.


 Please wait while we load your content...
Please wait while we load your content...