Dry reforming of methane over Co–Mo/Al2O3 catalyst under low microwave power irradiation†
Abstract
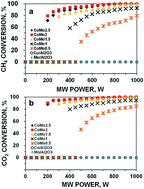
In this work, microwave (MW) irradiation was used to activate Co/Al2O3, Mo/Al2O3, and Co–Mo/Al2O3 catalysts for dry reforming of methane (DRM) reactions. Experimental results indicate that single metallic catalysts of either Co or Mo are inactive for DRM under all the tested conditions due to their limited MW-absorbing ability. In contrast, Co–Mo bimetallic catalysts supported by Al2O3 exhibit high catalytic activity due to the formation of a magnetodielectric Co0.82Mo0.18 alloy, which plays the dual role of a good MW acceptor and the provider of active centers for the DRM reaction. The MW power level required to activate such bimetallic catalysts for DRM is significantly dependent on the molar ratio between Co and Mo. The CoMo2 catalyst (with a molar ratio of 2.0 Co to 1.0 Mo) supported on Al2O3 exhibits the best catalytic performance, converting 80% CH4 and 93% CO2 to syngas at a ratio of H2/CO of 0.80 at the total volumetric hourly space velocity (VHSV) of 10 L g−1 h−1 and MW power of 200 W. As compared to the reported C-based catalysts, the Co–Mo/Al2O3 catalyst delivers more favorable stability over 16 time-on-stream (TOS) by virtue of its intrinsic ability to absorb MW without the inclusion of auxiliary MW acceptors.


 Please wait while we load your content...
Please wait while we load your content...