Effect of a ZrO2 support on Cu/Fe2O3–CeO2/ZrO2 catalysts for NO removal by CO using a rotary reactor
Abstract
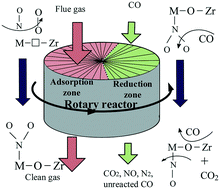
The effect of a ZrO2 support on the activity and adsorption behavior of Cu/Fe2O3–CeO2/ZrO2 catalysts was investigated by BET, XRD, SEM, Raman, H2-TPR and XPS. The NO removal performance of these catalysts in a rotary reactor at 150 °C was correlated with their catalytic activity for NO + CO, and the surface processes occurring upon NO + O2 interaction with the catalysts were studied by in situ DRIFTS. The results showed that the doping of suitable amounts of ceria into the zirconia lattice would lead to contraction of its lattice and an increase in bulk defects, which could promote the oxygen mobility and enhance the interaction between Cu/Fe2O3–CeO2/ZrO2 and ZrO2. The ZrO2 support could increase the concentration of oxygen vacancies, which could promote the dissociation of oxygen molecules and increase the activity for NO oxidation. The surface oxygen contributed to the conversion of NO2 into stable nitrates and the low concentration of surface oxygen promoted the formation of labile nitrites. The remarkably high NO removal efficiency was attributed to the significantly enhanced adsorption of unstable adsorbed NOx species (i.e., free nitrate ions and nitrosyl) and diminished stable adsorbed NOx species (i.e., bidentate nitrate and bridged nitrate) via Cu/Fe2O3–CeO2/ZrO2 support interactions.


 Please wait while we load your content...
Please wait while we load your content...