How do the unique Au/α-Fe2O3 interfacial structures determine activity in CO oxidation?†
Abstract
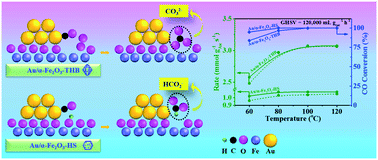
In this study, three α-Fe2O3 crystallites of regular morphology (truncated hexagonal bipyramid, quasi cubic, and hexagonal plate) were prepared in a controllable manner. Based on the (HR)TEM and SEM characterizations, the exposed crystal facets of three α-Fe2O3 crystallites, namely {113}, {214}, {104}, {110}, {012}, and {001}, were carefully identified. Au nanoparticles of ca. 2.0 nm with a narrow particle size distribution were essentially monodispersed on the three α-Fe2O3 substrates through a controlled deposition strategy. In such a way, the Au/α-Fe2O3 interfacial structures with structurally defined oxide substrates and nearly identical Au particle size and morphology have been obtained. The systems allowed us to compare in depth the behaviors of distinct surfaces/interfaces in CO oxidation. The characterization including O2/surface hydroxyl-TPD, CO-TPSR, and in situ FTIR clarified the role of the surface oxygen/hydroxyl species in developing crucial intermediates on distinct interfaces. The results demonstrated that the evolution of different intermediates (CO32− and HCO2−) was directly controlled by interfacial features, i.e., the weakly adsorbed oxygen and surface hydroxyl species as well as the specific Au–Fe2O3 boundary structure, which meaningfully determined CO activation and conversion to CO2. The present study provided new insights into the significance of Au/α-Fe2O3 interfacial structures governing the evolution of reaction intermediates in CO oxidation.


 Please wait while we load your content...
Please wait while we load your content...