Engineering P450LaMO stereospecificity and product selectivity for selective C–H oxidation of tetralin-like alkylbenzenes†
Abstract
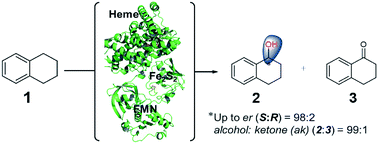
The P450-mediated asymmetric hydroxylation of inert C–H bonds is a chemically challenging reaction. Self-sufficient P450LaMO from the CYP116B subfamily could catalyze the transformation of 1,2,3,4-tetrahydronaphthalene to (S)-tetralol, despite its poor enantioselectivity (er 66 : 34) and product selectivity (the ratio of alcohol and ketone, ak, 76 : 24). To improve the selectivity, phenylalanine scanning and further protein engineering were performed to reshape the active pocket of P450LaMO, resulting in a mutant (T121V/Y385F/M391L) with not only improved (S)-enantioselectivity (er 98 : 2) but also excellent product selectivity (ak 99 : 1), in contrast to another mutant L97F/T121F/E282V/T283Y with complementary (R)-enantioselectivity (er 23 : 77). Moreover, the enantiopure (S)-alcohols formed by the P450LaMO-catalyzed oxidation of a series of alkylbenzenes are potentially important building blocks in the pharmaceutical industry. This Phe-based enantioselectivity engineering used for reshaping the active pocket of P450s could provide a guide to the protein evolution of other CYP116B members.


 Please wait while we load your content...
Please wait while we load your content...