Reduction of NO with NH3 over ferric oxide nanocrystals: the crystallographic facet-induced catalytic enhancement†
Abstract
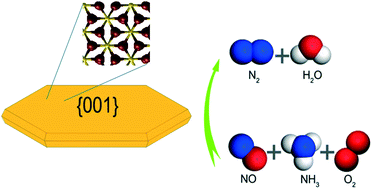
Different synthesis methods of a given material will lead to different mesoscopic architectures, which in turn would strongly influence the catalytic performance. Therefore, morphology control is thought to be helpful for creating high-performance catalysts. Here, a series of well-defined α-Fe2O3 crystals with different morphologies, including hexagon, rod, and diamond, have been prepared and investigated for the selective catalytic reduction (SCR) of NO with NH3, which is of major environmental interest for air pollution control. We report that the {001} surface of the hexagon-shaped Fe2O3 catalyst is very active for NO removal. From temperature-programmed desorption measurements, we find that the NH3 reactant adsorbed strongly on the {001} surface. In contrast, other high-index ferric oxide facets, including {012}, {−210}, {01−4}, {110}, and {1−10}, exhibit the weakly adsorbed state of NH3. Density functional theory calculations show that the {001} surface has a high surface energy of 1.4 J m−2, which should account for the observed SCR kinetics. A link between the experimentally observed activity trend and the exposed facets of ferric oxides has been established.


 Please wait while we load your content...
Please wait while we load your content...