Synthesis of Co–Mn oxides with double-shelled nanocages for low-temperature toluene combustion†
Abstract
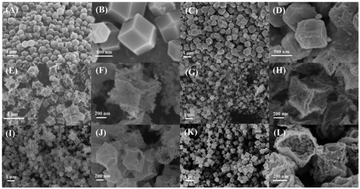
In this paper, we report a template-engaged strategy for the synthesis of Co–Mn oxides with double-shell box-in-box hollow nanocage (BHNC) structures. The inner shell of the box is made of Co3O4, while the outer shell is made of Co–Mn oxides. The composition of the outer shell was tuned by changing the Mn/Co molar ratio. This facile method can be used to construct box-in-box hollow structures of other transition metal oxides such as those of Co–Ni, Co–Cu and Co–Fe. These Co–Mn oxides with BHNCs exhibit toluene conversions over 90% at 250 °C, which is much higher than those of Co3O4 nanocages (NCs) and the traditional Co3O4-CP synthesized by co-precipitation method at the same temperature. Moreover, the former is superior to the latter in terms of resistance towards H2O and CO2. The superior catalytic performance of Co1Mn1 BHNCs is attributed to the high mobility of lattice oxygen, low toluene desorption temperature and active Co3+ sites originated from the oxidation of Co2+ by Mn3+.


 Please wait while we load your content...
Please wait while we load your content...