Synthesis of higher alcohols by CO hydrogenation on a K-promoted Ni–Mo catalyst derived from Ni–Mo phyllosilicate†
Abstract
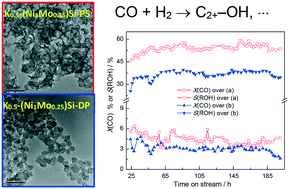
Higher alcohols (HAs) with carbon numbers exceeding two (C2+–OH) can be used as environmentally friendly gasoline additives, clean fuels, and chemical products. The synthesis of HAs from syngas is a highly important branch in C1 chemistry. However, design of catalysts with high selectivity to C2+–OH and long operating stability remains a challenge. Here, a K-promoted Ni–Mo catalyst [K-(NiMo)Si-PS] derived from Ni–Mo phyllosilicate (PS) was fabricated by an ammonia evaporation method and tested for the synthesis of HAs by CO hydrogenation under the reaction conditions of 3.0 MPa and 240 °C. The optimal K-(NiMo)-PS catalyst can give 43.8% selectivity to HAs (65.4% selectivity to C2+–OH in total alcohols), with stable activity for 200 h, and markedly outperformed catalysts with the same composition prepared by conventional co-deposition and wet impregnation methods. A series of spectroscopic characterizations indicated that the superior performance of K-(NiMo)Si-PS can be attributed to the enhanced active metal–support interactions inherited from the PS precursor, resulting in an increased active metal dispersion with abundant Mo3+ and NiO(OH) species on catalyst surfaces. The findings revealed that using Ni–Mo PS is an effective alternative in preparing catalysts for the synthesis of HAs by CO hydrogenation.


 Please wait while we load your content...
Please wait while we load your content...