Tuning the Pd-catalyzed electroreduction of CO2 to CO with reduced overpotential†
Abstract
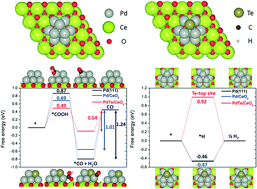
Developing selective and efficient catalysts is highly desirable for electrochemical CO2 reduction (ECR) to fuels and chemicals. Pd can strongly bind *COOH but weakly bind *CO, thus resulting in CO as a product. However, proton reduction also occurs severely on the surface of Pd, leading to low CO selectivity. Here we found that the ECR to CO can be greatly enhanced by controlling the Pd–ceria interface and doping with tellurium atoms. Notably, a very high mass activity of 92 mA mgPd−1 (at 1.0 V vs. reversible hydrogen electrode) for CO formation was achieved, significantly surpassing previously reported Pd catalysts (35 mA mgPd−1 at −1.0 V). The Pd catalysts comprising CeOx displayed more positive onset potentials than the Pd catalysts in the absence of CeOx, enabling ECR to CO at −0.6 V (vs. RHE). The modified Pd catalyst also afforded an unprecedented CO faradaic efficiency of over 84% at a low Pd loading (<3 wt%). Density functional theory calculations revealed that the Pd atoms located between the Te dopant and CeO2 promoted CO formation, thus improving CO2 conversion efficiency.


 Please wait while we load your content...
Please wait while we load your content...